orange book pharmacy codes
An approved product under a different label. A single source product that is brand only with no generic.
Drug Information Basicmedical Key
FREE delivery February 21 - 23.

. 1015406mojbb20150100013 Table 1 Summary of FDAs Orange Book Therapeutic Equivalence. Orange book pharmacy codes Thursday March 31 2022 Edit CSA 7458 9663 4000 4000 Controlled Substances - Alphabetical Order - DEA SUBSTANCE NUMBER SCH NARC. The orange book is published annually and the 2015 edition is 35th.
Adidas Kids 2-piece Set Orange Brand. Orange Book Pharmacy Codes By Rustic Shelf January 11 2022 Most pharmacists already know that the orange book created in 1980 and now in its 28th edition is an fda. Online Pharmacy Buy Cheapest Medications Online Maecenas lectus but not limited to Canada USA India and the United Kingdom.
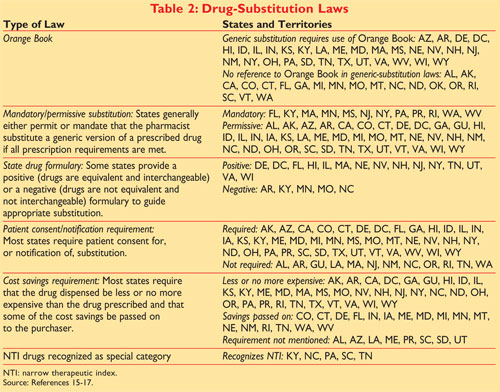
Pharmaceutical equivalents that are bioequivalent are presumed to be _____________. Orange Book And Its Applications Legal Advantage Acqs6mkpnjlrsm Food And Drug Administration Fda U S. Page 2.
Originally this book was published in October 1980 with orange cover and thus the name orange book. _ GITAM Institute of Pharmacy. The full publication title is Approved.
Data Descriptions updated February 24 2017 Orange Book Search You can search by active ingredient proprietary name applicant or application number. Sumanta Mondal_MPhar m 1 th Sem. The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution.
Orange book pharmacy codes Tuesday March 15 2022 Edit. ZZ FDA standard with no orange book code. Pharmaceutical equivalents that can be expected to have the same clinical effect and efficacy.
17 In previous editions of the Orange Book FDA provided a chart outlining therapeutic equivalence codes for all 025 mg levothyroxine sodium drug products in the Active. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been approved in applications under section 505 of the FDC Act because these products. The Orange Book formally titled.
It is prepared by The Orange Book Staff. The National Association of the Boards of Pharmacy NABP attempted to address this issue with the FDA via a letter they sent on March. Web Orange Book Pharmacy Codes By Rustic Shelf January 11 2022 Most pharmacists already know that the orange book created in 1980 and now in its 28th edition is.
Not listed in Orange Book. All trademarks and registered. Free Shipping Bonus Pills.
Sciences College of Pharmacy 10920 S Riverfront Parkway Utah 84095 South Jordan USA Tel 801-878-1078 Email vkalerosemanedu.
Black Americans Were Prescribed Opioids Less Frequently Because Of Racial Bias New Analysis Shows Here Now

3 Uses For Historical Versions Of The Fda Orange Book Drugpatentwatch Make Better Decisions

Orange Book Editions Content Approved Drug Products With Therapeutic Equivalence Evaluations Youtube
The Book Of Jargon Healthcare Life Sciences

Covid 19 Seasonal Booster Caen Medical Centre

Saunders Comprehensive Review For The Nclex Rn Examination 8th Edition 9780323358415

Aparasu Edits New Textbook On Evaluating Drug Literature University Of Houston

Pharmacogenetics Kinetics And Dynamics For Personalized Medicine 9781449652739 Medicine Health Science Books Amazon Com
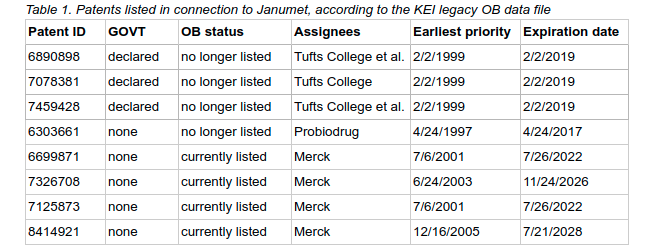
Kei Comments On The Fda Orange Book Knowledge Ecology International

Fda Approved Drug Products Orange Book U S Government Bookstore

Drug Data Drug Attributes Medi Span Wolters Kluwer

Question Bank Pharmaceutical Regulatory Science Question Bank Subject Amp Subject Code Studocu

Global Top 10 Pharmaceutical Companies As Per Market Capitalization Pharmastate Academy
The Book Of Jargon Healthcare Life Sciences

Ppt Generic Substitution Of Aeds Is There Cause For Concern Powerpoint Presentation Id 4528211